What is a chemical equation?
A chemical equation is a symbolic representation of a reaction where the compounds written on the left side of the arrow are reactants and the compounds written on the right side of the arrow are products.
For example, it can be represented as:
2H2 + O2 → 2H2O
H2 and O2 are the reactants and H2O is the product.
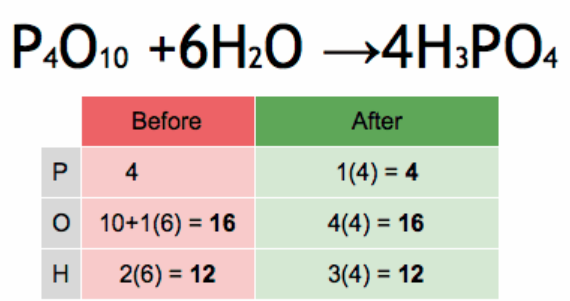
What is a balanced chemical equation?
A balanced chemical equation is an equation of a chemical reaction where the number of atoms of each element in the reaction as well as the total charge are the same for both the products and the reactants. The charge and the mass are balanced on both sides of the chemical reaction. It is also known as the conservation of charge and mass or balancing of the reaction.
Example of a balanced chemical equation
Consider the following reaction:
2 Fe2O3 + 3 C → 4 Fe + 3 CO2
The right and left sides of the equation have 4 iron, 6 oxygen, and 3 carbon atoms. Whenever you are balancing equations, always multiply the subscript of every atom by the coefficient. If in case there is no subscript, consider its value as 1.
You can also denote the state of matter of each compound of reactant. It is usually written in parentheses after the compound. The above reaction is written as:
2 Fe2O3(s) + 3 C(s) → 4 Fe(s) + 3 CO2(g)
where s denotes a solid and g refers to a gas
Unbalanced chemical equation
An unbalanced chemical equation can be defined as the number of molecules of atoms present on the left side of the arrow are not equal to the number of molecules of atoms present on the right side of the arrow.
For instance, consider the following equation:
Fe2O3 + C → Fe + CO2
This equation is unbalanced with respect to mass whereas it is balanced for charge.
The equation has 2 Fe atoms on the left side of the arrow and 1 Fe atom on the right side of the arrow. The aim of balancing the chemical equation is to equal the number of each type of atom in reactant and product.
We can achieve this by changing the coefficients (numbers written in front of each compound). Remember that the subscripts cannot be changed. Subscripts are the numbers written to the right side of some atoms.
To learn more about various other chemistry topics such as types of chemical reactions, Functional groups and more visit BYJU’S.
Also, you can subscribe to our BYJU’S YouTube channel for a better understanding of the concepts.